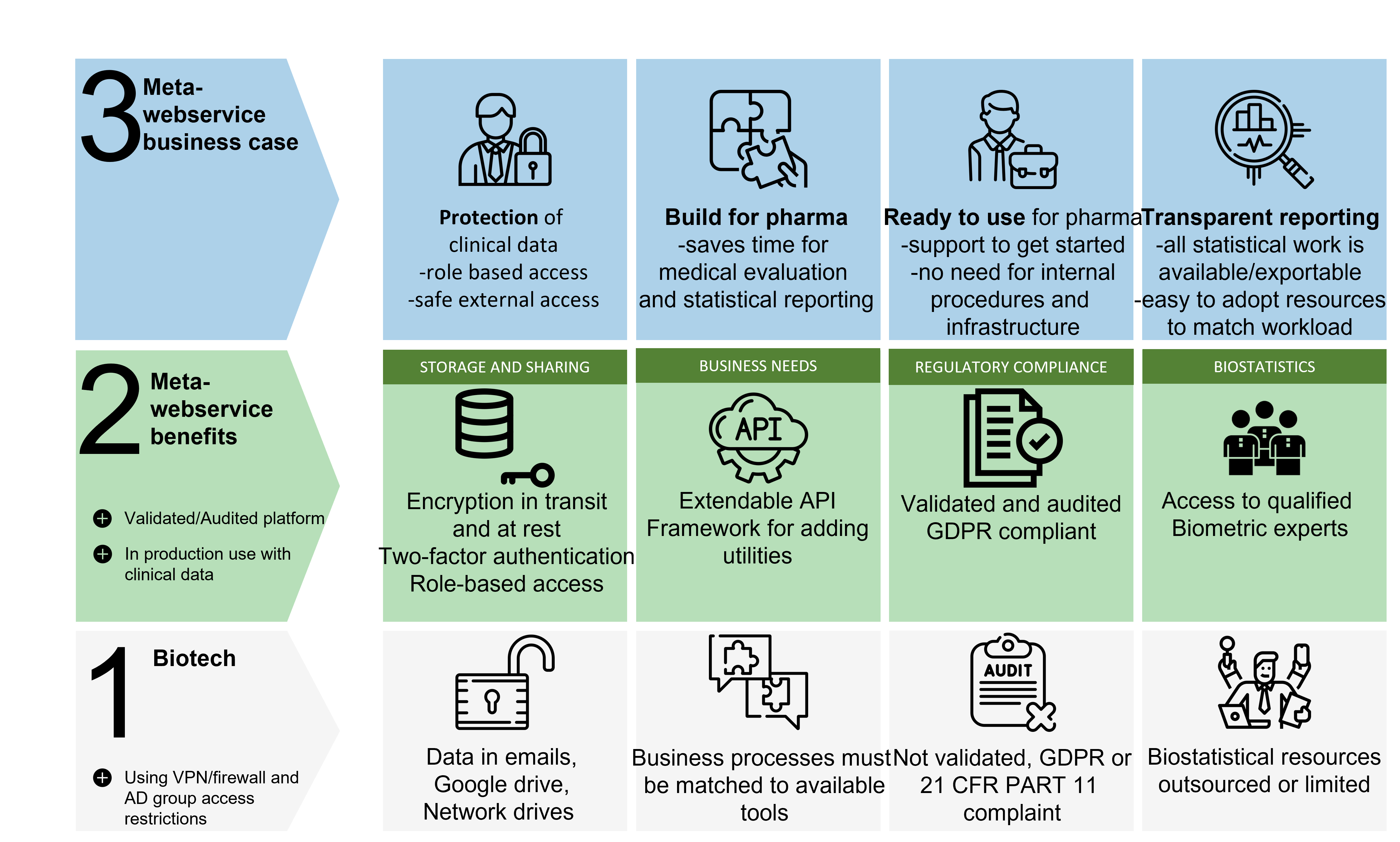
CRO services via Metawebservice portal
We utilise the Metawebservice portal to achive our value proposition.
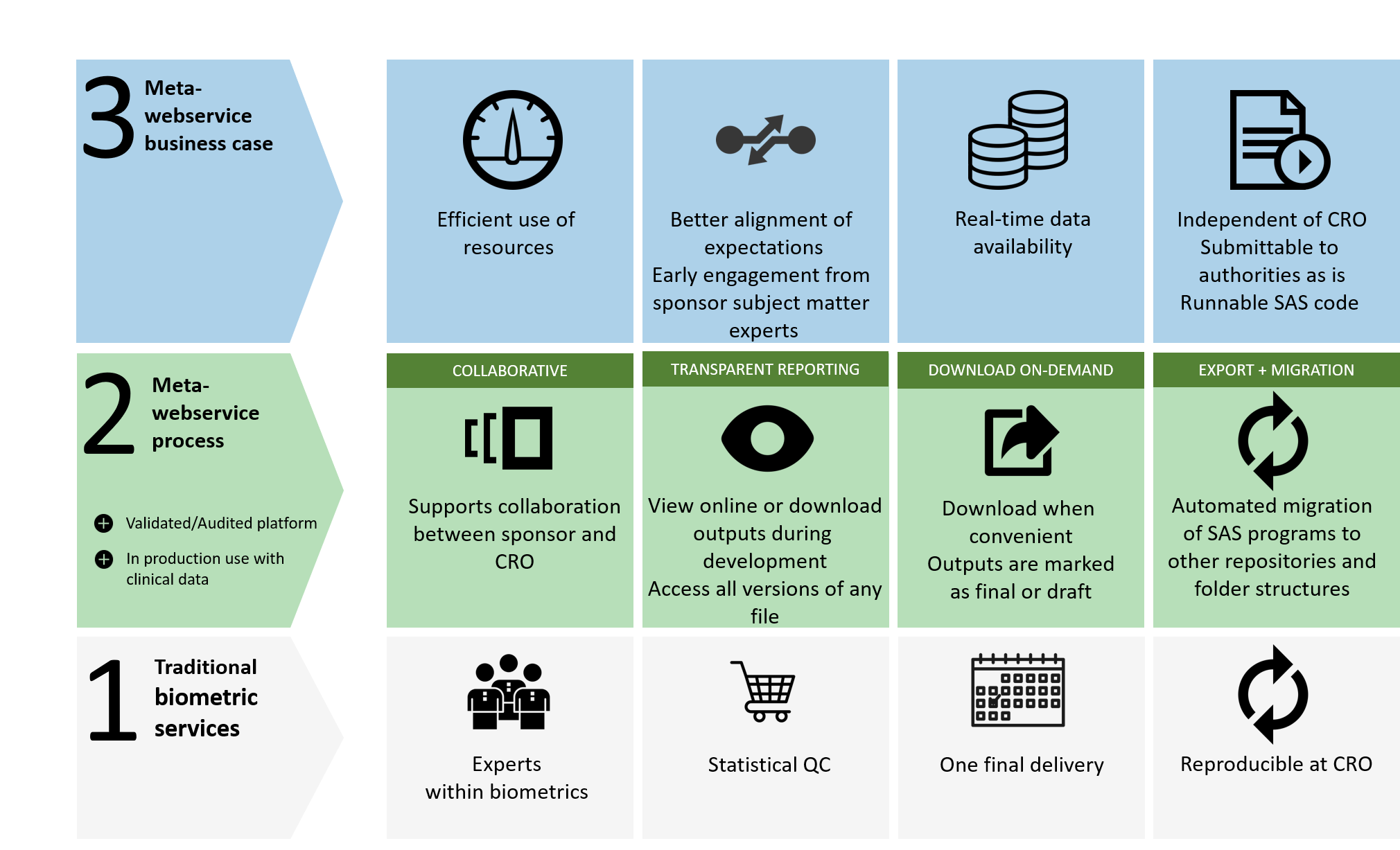
Why use ME-TA?
Utilises the Metawebservice platform to enable a closer collaboration with a sponsor.
Extensive experience (Worked for 5 of the 10 largest pharma companies).
Innovative mindset.